SELECTED RECENT PUBLICATIONS
For a full list of Khavari lab publications please click here
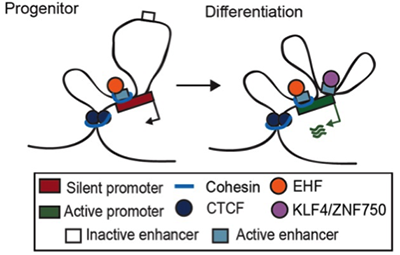
Lineage-specific Dynamic and Pre-established Enhancer-Promoter Contacts Cooperate in Terminal Differentiation.
Rubin AJ, Barajas BC, Furlan-Magaril M, Lopez-Pajares V, Mumbach MR, Howard I, Kim DS, Boxer LD, Cairns J, Spivakov M, Wingett SW, Shi M, Zhao Z, Greenleaf WJ, Kundaje A, Snyder M, Chang HY, Fraser P, Khavari PA. NATURE GENETICS (2017).
A kinetic study of 3D chromatin dynamics across the genome of normal progenitor cells undergoing terminal differentiation identified two types of transcriptional enhancers in contact with target genes. Constitutive enhancers are pre-looped, H3K27ac-marked, and cohesin-bound while dynamic enhancers gain H3K27ac activation marks and loop to target genes only during differentiation. Distinctive sets of transcription factor (TF) bind each enhancer class and this work discovered EHF as a new essential TF required for constitutive enhancer function in this setting. These new features of genome regulation during stem cell differentiation underscore the diversity of mechanism engaged during this process.
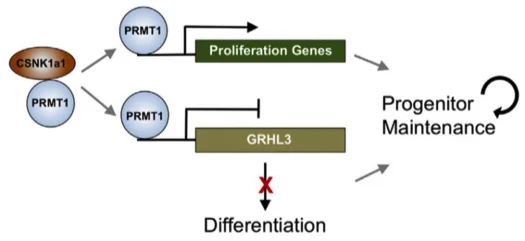
CSNK1A1 REGULATES PRMT1 TO MAINTAIN THE PROGENITOR STATE IN SELF-RENEWING SOMATIC TISSUE.
Bao, X, Siprashvili Z, Zarnegar BJ, Shenoy, RM, Rios, EJ, Nady N. Qu, K, Mah, A, Webster, DE, Rubin, AJ, Wozniak, GG, Tao, S, Wysocka, J, Khavari PA. DEVELOPMENTAL CELL (2017).
Screening for histone modifiers essential for progenitor maintenance demonstrated that the PRMT1 histone arginine methyltransferase is required to sustain progenitor cells in the undifferentiated state in vivo in both murine and human epidermis. LC-MS/MS of PRMT1 associated proteins demonstrated that the CSNK1a1 kinase directly binds PRMT1 and controls its genomic targeting. PRMT1 and CSNK1a1 act to cooperatively suppress the pro-differentiation transcription factor, GRHL3, thereby preventing differentiation. These data identify partners and targets of arginine methyltransferases in histone modification and genome during progenitor maintenance.
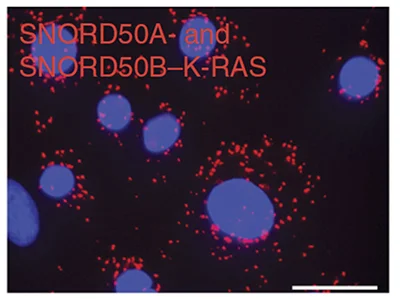
The noncoding RNAs SNORD50A and SNORD50B bind K-Ras and are recurrently deleted in human cancer.
Siprashvili Z, Webster DE, Johnston D, Shenoy R, Ungewickell A, Bhaduri A, Flockhart R, Zarnegar BJ, Che Y, Meschi F, Puglisi JD & Khavari PA. NATURE GENETICS (2016).
To help define roles for specific small noncoding snoRNAs in cancer, we analyzed 5,473 pairs of tumor and matching normal genomes in 21 human cancer types to discover recurrent deletion of SNORD50A/B in 26% of human tumors. CRISPR knockouts in tumor cells combined with new methodologies to analyze RNA-protein interactions characterized SNORD50A/B as a Ras-binding tumor suppressor RNA that inhibits Ras function by altering its post-translational modification. Small noncoding RNAs therefore can play dominant roles in cancer by binding oncogenes and altering their function.
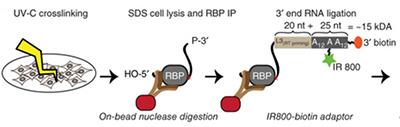
irCLIP PLATFORM FOR EFFICIENT CHARACTERIZATION OF PROTEIN–RNA INTERACTIONS.
Zarnegar, BJ, Flynn RA, Shen Y, Do BT, Chang HY & Khavari PA. NATURE METHODS (2016).
The complexity of transcriptome-wide protein-RNA interaction networks is incompletely understood, with current methods resource intensive and technically challenging, irCLIP provides an untraefficient, fast, and nonisotopic method for the detection of protein-RNA interactions.
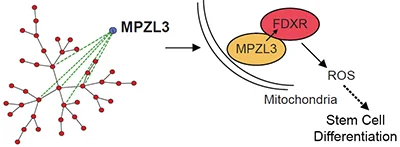
Network Analysis Identifies Mitochondrial Regulation of Epidermal Differentiation by MPZL3 and FDXR.
Bhaduri A, Ungewickell A, Boxer LD, Lopez-Pajares V, Zarnegar BJ, Khavari PA. DEVELOPMENTAL CELL (2015).
Network reconstruction approaches can help discover new regulators of stem cell differentiation. We generated Proximity Analysis to apply topologically constrained scale-free, small-world biological network construction to discover an essential role for MPZL3, FDXR and reactive oxygen species in epidermal differentiation.
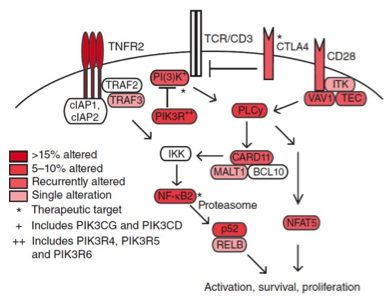
Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2.
Ungewickell A, Bhaduri A, Rios E, Reuter J, Lee CS, Mah A, Zehnder A, Ohgami R, Kulkarni S, Armstrong R, Weng WK, Gratzinger D, Tavallaee M, Rook A, Snyder M, Kim Y & Khavari PA. NATURE GENETICS (2015).
Cancer is characterized by enhanced cellular proliferation in concert with increased resistance to cell death. The mechanisms whereby stem cells undergoing malignant transformation achieve these dual aberrations, however, are highly diverse, depending on tissue and tumor type. Here we use high throughput sequencing to identify recurrent induction of cell survival and activation pathways in cutaneous T cell lymphoma.
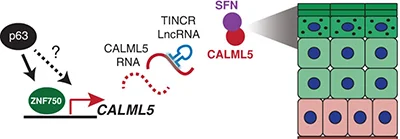
CALML5 is a ZNF750- and TINCR-induced protein that binds stratifin to regulate epidermal differentiation.
Sun BK, Boxer LD, Ransohoff JD, Siprashvili Z, Qu K, Lopez-Pajares V, Hollmig ST, Khavari PA. GENES & DEVELOPMENT (2015).
Stem cell differentiation in solid tissue requires spatially coordinated gene regulation. We used LCM RNA-seq to identify CALML5 as the most highly upregulated gene in differentiated epidermis and an essential regulator of this process. The TINCR lncRNA and the ZNF750 TF controlled CALML5 levels . LC-MS/MSidentified SFN as a key CALML5 partner essential for late differentiation.
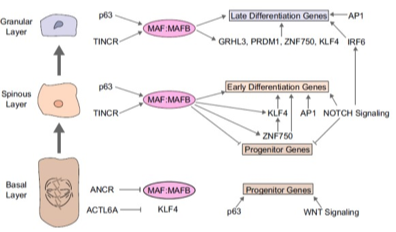
A LncRNA-MAF:MAFB transcription factor network regulates epidermal differentiation.
Lopez-Pajares V, Qu K, Zhang J, Webster DE, Barajas BC, Siprashvili Z, Zarnegar BJ, Boxer LD, Rios EJ, Tao S, Kretz M & Khavari PA. DEVELOPMENTAL CELL (2015).
Precisely orchestrated stem cell differentiation is critical for homeostasis in epidermis and other tissues, however, the regulatory networks involved in this process are incompletely understood. Here we identify a new network that is essential for spatially precise genome regulation during epidermal differentiation. At the center of this network are the MAF and MAFB transcription factors (TFs), which are regulated by the TINCR and ANCR lncRNAs to control key effector TFs, including GRHL3, PRDM1, ZNF750 and KLF4.
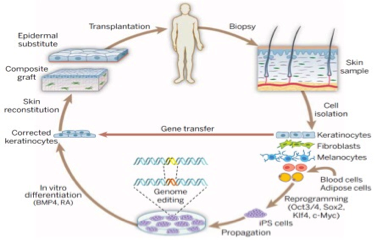
Advances in skin grafting and treatment of cutaneous wounds.
Sun BK, Siprashvili Z & Khavari PA. SCIENCE (2014).
Tissue regeneration after injury is essential for survival. Advances in stem cell biology, genome editing, and tissue regeneration have laid the foundation for new approaches to cutaneous regeneration and grafting. Cas9 gene-edited somatic cells and iPS cells may be used in this process for the treatment of a host of monogenic disorders, such as epidermolysis bullosa (EB).
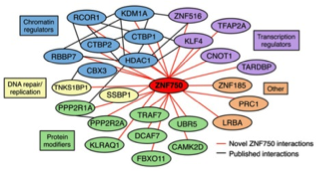
ZNF750 interacts with KLF4 and RCOR1, KDM1A, and CTBP1/2 chromatin regulators to repress epidermal progenitor genes and induce differentiation genes.
Boxer LD, Barajas B, Tao S, Zhang J & Khavari PA. GENES & DEVELOPMENT (2014).
Stem cell differentiation requires down-regulation of stem cell gene expression and induction of terminal differentiation genes. Dominant TFs, such as ZNF750, accomplish both types of impacts, but how they simultaneously effect selective gene repression and activation is unclear. Here we characterize the ZNF750 protein interactome by LC-MS/MS to discover that ZNF750 targets KDM1A to repress stem cell genes while simultaneously co-binding with KLF4 at differentiation genes to activate their expression.
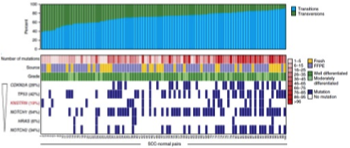
Recurrent point mutations in the kinetochore gene KNSTRN in cutaneous squamous cell carcinoma.
Lee CS, Bhaduri A, Mah A, Johnson WL, Ungewickell A, Aros CJ, Nguyen CB, Rios EJ, Siprashvili Z, Straight A, Kim J, Aasi SZ & Khavari PA. NATURE GENETICS (2014).
Epithelial neoplasms, which comprise ~90% of human cancers, are characterized by widespread genome damage by mechanisms that are incompletely understood. Here we used high throughput genome sequencing to define recurrent mutations in epidermal squamous cell carcinoma (SCC), the second most common cancer in humans. SCC displays hotspot mutations in the KNSTRN gene that trigger aneuploidy and accelerate tumorigenesis, identifying a new common mechanism of genomic injury in cancer.